Rational Design of Oncolytic Viruses
In this post we give an overview of the history of using viruses as potential treatment for cancer. We also outline the challenges a great viral therapy needs to overcome. Last, we review our approach to rationally design and engineer safe and effective oncolytic viruses.
History and current state
Since the middle of the last century there has been anecdotal evidence that virus infections can help in curing or slowing down cancer. Unfortunately these infections happened randomly and the results were hard to reproduce. This spurred the first wave of research, trying naturally occurring viruses as cancer therapeutics. Even the Rabies virus has been tried.
It has become increasingly clear that there is no virus known to us in nature which can be used as a reliable cancer therapy. However, with the increased knowledge about virology and oncology a great viral therapy could be designed and engineered.
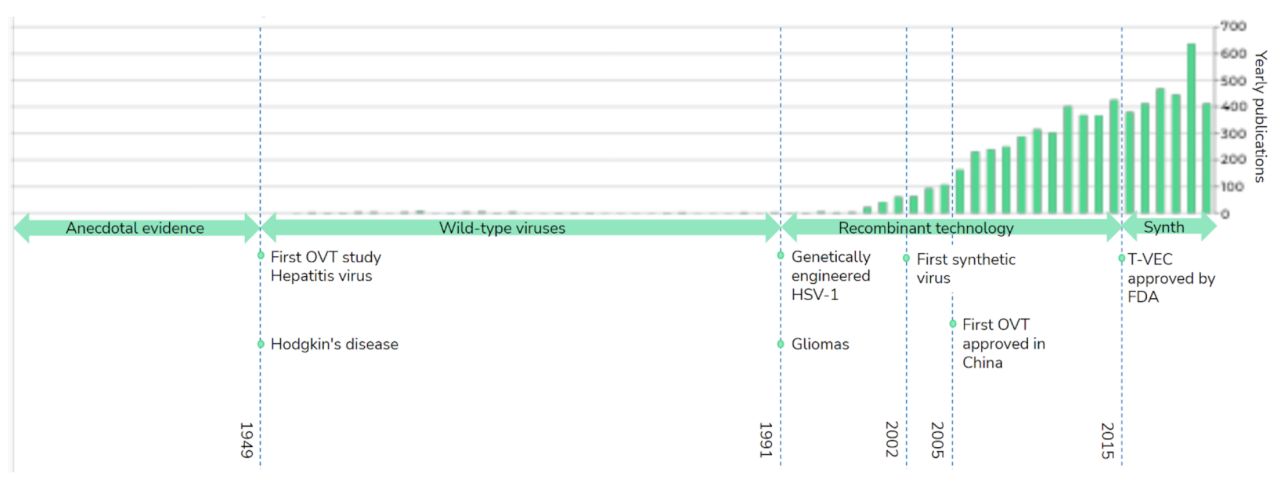
In the 1990s DNA editing techniques using restriction enzymes became accessible and enabled the first attempts to improve natural viruses and engineer oncolytic viruses. In 2005 the first OVT, based on a modified Adenovirus, was approved in China for the treatment of head and neck squamous cell carcinoma. More recently, in 2015 a modified Herpes Simplex virus, T-VEC, for the treatment of melanoma, was approved by the FDA in the USA. At the same time the amount of publications about OVTs has rapidly increased, showing a great scientific interest in the field. There are currently 145 studies registered on clinicaltrials.gov. The outcomes of these studies have shown oncolytic virus therapy is generally safe, but therapeutic efficacy has fallen short of expectations. Why is that the case? Simply put, the amount of changes needed to make a natural virus work well as an OVT are considerable. The tools used and available are complicated, error prone, limited in design freedom and time consuming.The result is, that using the conventional approaches it takes a lot of time and money to make relatively small changes reliably. For this reason, we set out to build a platform, using our own DNA editor with advanced algorithms, DNA synthesis and advanced assembly approaches, to make engineering of OVT much easier, with complete design freedom, more precise and at a much lower cost.
Let’s have a look at what we believe are the best characteristics of an OVT.
Ideal Oncolytic Virus
The ideal oncolytic virus therapy is non-pathogenic and cancer-specific, has high lytic effect, has low or no seroprevalence, supports transgene insertion and expression, and is genetically stable.
Non pathogenic and cancer specific means that the virus will not cause disease and it only kills cancer cells (or causes cancer cells to be killed). Specificity is typically achieved by the matching of the glycoprotein and the specific receptor on the cell. If the receptor on the cell is abundantly present on the cancer cell and rarely present on healthy cells this presents an opportunity for a specific therapeutic. Through research and the results from therapies targeting a single marker, we are learning however, that any highly expressed receptor on cancer cells is also present on (too) many healthy cells. Therefore a second condition needs to be met before the therapeutic effect should start. With oncolytic viruses, the therapeutic effect is based on the replication of the virus. It is possible to create conditionally replicating viruses using genetic elements such as genetic switches, promoters and aptazymes.
High lytic effect means that the virus quickly replicates in the right conditions. It has proven to be challenging to engineer a virus that is both highly lytic and safe.
No seroprevalence means that the patient has no existing immunity. It allows for the virus to be administered systemically in relatively low dosage and have enough time to reach and lyse cancer cells before the patients’ immune system reacts. If the patient would have existing immunity, then this amount of time would be reduced and it would be ineffective to administer systemically. Systemic administration is preferred, because it allows for the treatment of hard to reach tumors and will also treat (micro)metastasis.
Transgenes are genes not native to the virus that can cause expression of proteins, which help the virus in addressing other challenges. An example would be the penetration of the extracellular matrix (ECM) surrounding solid tumors.
Genome stability refers to the mutation rate or changes in the viral genome. As a result of mutations, antitumor activity and efficacy may be attenuated, or safety may be compromised as the virus adapts and begins infecting healthy cells.
Rational Design of oncolytic viruses
The ideal oncolytic virus does not seem to exist in nature, so we have set ourselves the mission to design and engineer it. We believe that our platform is the tool we need to make this happen.
A great starting point for a rationally designed virus requires meeting all of the criteria mentioned above. Vesicular stomatitis virus (VSV) possesses these characteristics and thus serves as a great candidate. It is non-pathogenic and since most people have never been exposed to it, it has no seroprevalence. VSV can be retargeted to facilitate selective infection and its genomic structure is well understood. As such, extensive engineering is relatively easy. The biggest challenge is to make it selectively replicating in the right environment. For DNA viruses this can be done using promoters, which are well understood. Since VSV is a RNA virus we need to use aptazymes or other genetic circuits. Early data indicates that we have successfully created a virus with a functional aptazyme (“ON” switch).
Using our platform, we have designed and made a virus based on the criteria above, that selectively infects and replicates in liver cancer cells. In combining both selective infection and selective replication, it shows very good safety characteristics. Still, it replicates fast and shows great efficacy. Our initial data is showing, at least in cells (in vitro), that we have created a great therapeutic virus for liver cancer. We are currently testing it extensively, both in cells and very soon in mice.
We hope and believe that we are capable to rationally design and engineer great therapeutic viruses to be used as cancer therapeutics.